What is the secret of the beautiful colors that we see during fireworks?
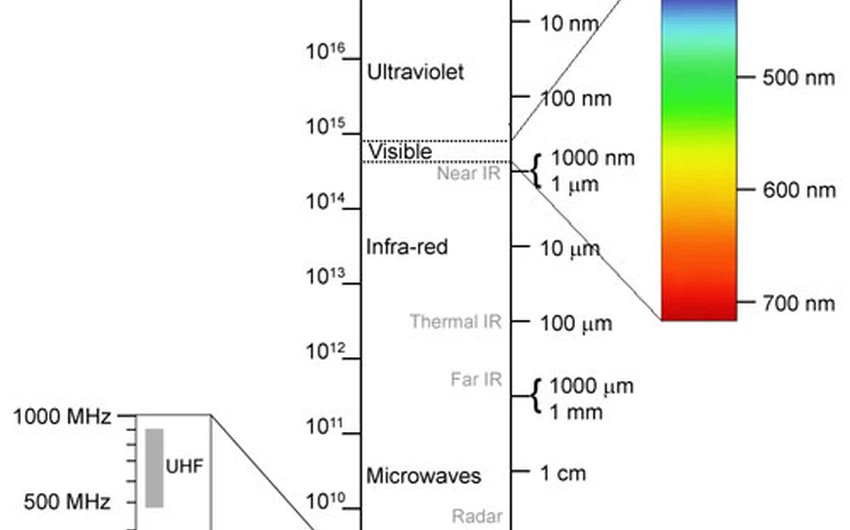
<p style=";text-align:left;direction:ltr">ArabiaWeather.com – Many people around the world celebrate fireworks because of their aesthetics and pleasure when watching them in the sky, but did anyone wonder how these red, orange, yellow, green and blue colors appear in this way?</p><p style=";text-align:left;direction:ltr"> <span style="line-height: 1.6em;">Fireworks consist mainly of gunpowder salts; Where salt is scientifically defined as an ionic mixture formed as a result of the interaction between acids and bases, which gives a basic characteristic of salts in the dry state that they are not able to transmit electricity.</span></p><p style=";text-align:left;direction:ltr"></p><p style=";text-align:left;direction:ltr"> The gunpowder salts used in fireworks consist of many types: strontium carbonate (which is responsible for the appearance of the red color in the sky), calcium chloride (the appearance of orange), sodium nitrate (yellow color), barium chloride (green color) and copper chloride (the colour blue). It is also possible to obtain several colors mediating these basic colors when these salts are mixed with each other in certain proportions.</p><p style=";text-align:left;direction:ltr"> For example, the violet color appears in the sky as a result of the interaction of strontium salts (which are responsible for the red color) with copper chloride salts (the blue color).</p><p style=";text-align:left;direction:ltr"></p><p style=";text-align:left;direction:ltr"> And when going into more detail regarding the gradation of visible colors in the sky; We find that these salts, when burned, emit spectra with specific wavelengths; The blue color appears when waves are emitted from copper chloride salts with a length of between 400-500 nanometers, which is the shortest wavelength within the visible rays of the human eye, while the strontium salt emits wavelengths of 600-700 nanometers, where we see then the red color, which is the longest visible wavelength to the human eye.</p><p style=";text-align:left;direction:ltr"></p><p style=";text-align:left;direction:ltr"> <strong>See also:</strong></p><p style=";text-align:left;direction:ltr"> <strong style="line-height: 1.6em;"><a href="http://www.arabiaweather.com/content/إطلاق-300-صاروخ-ألعاب-نارية-إلى-الأجواء-دفعة-واحدة">Launching 300 fireworks into the air at once</a></strong></p><p style=";text-align:left;direction:ltr"> <strong><a href="http://www.arabiaweather.com/content/مغامر-يجعل-مدينة-كاملة-تعتقد-بأن-نيزكاً-سقط-عليهاشاهد-ماذا-فعل">An adventurer makes an entire city believe that a meteorite has fallen on it..Watch what he did</a></strong></p>
Arabia Weather App
Download the app to receive weather notifications and more..